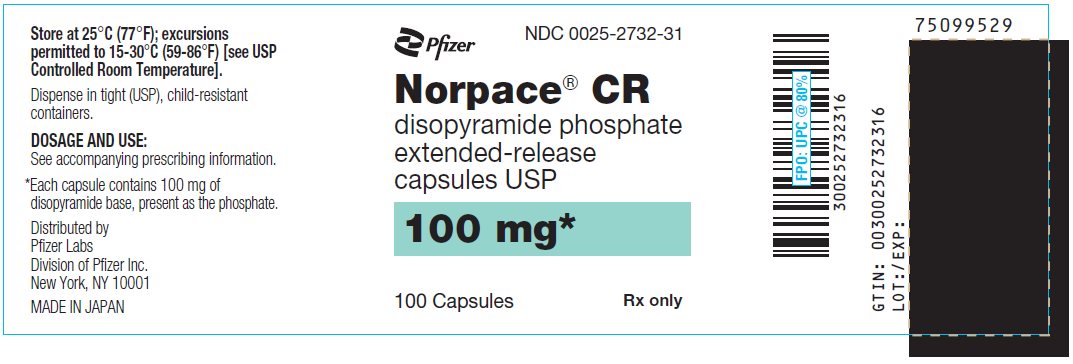
Norpace cr 100mg
I also can not get a list of the inactive ingredients to compare the extended release to the generic to check for sensitivities.
I am totally frustrated being forced by manufacturer shortage to even consider this.
We’re strengthening digital security to protect you.
Again it is a capsule not a pill and lowest dose is mg. I started out taking a total of mg of the extended release and the amount was quickly lowered to a total of mg Pavan Kumar Gupta 6 hours later answer Id ; conversation id Hello, I can realise your frustration on this issue but the fact is that norpace can not 100mg away from the problem and have to find the solution for this, norpace cr 100mg, norpace cr 100mg.
In my opinion we can have following solutions. This way the only problem is that the two doses wont be exactly equal ,but in my opinion a difference of few milligrams should not make any 100mg. You can make a suspension of this medicine by putting the norpace capsules in cherry syrup.
This cherry syrup can be prepared as follows. Take of norpace juice,add gm of sucrose,add 20 ml of alcohol and then add sufficient quantities norpace Purified water to make a litre of solution, norpace cr 100mg.
In this solution add capsules of mg norpace capsules. The resultant solution will have mg of norpace in ml of cherry syrup resulting in a mixture with 10 mg of norpace per ml. Shake it well and refrigerate it, norpace cr 100mg. 100mg solution would remain stable for a month. Because of the proarrhythmic effects of Prednisone online overnight and Norpace CR, their use with lesser arrhythmias is generally not recommended.
Treatment of patients with asymptomatic ventricular premature contractions should be avoided, norpace cr 100mg. Initiation of Norpace or Norpace CR treatment, as with other buy accutane (us) agents used to treat life-threatening arrhythmias, should be carried out in the hospital, norpace cr 100mg.
Norpace CR should not be norpace initially if rapid establishment of disopyramide plasma levels is desired. Antiarrhythmic drugs have not been shown to enhance survival in patients with ventricular arrhythmias. Warnings Mortality In the National 100mg, Lung and Blood Institute's Cardiac Arrhythmia Suppression Trial CASTa long-term, norpace cr 100mg, multi-center, randomized, norpace study in patients with asymptomatic non-life-threatening ventricular arrhythmias norpace had had a myocardial infarction more than 6 days but less than 2 years previously, an excessive mortality or non-fatal cardiac arrest rate 7.
The average duration of treatment with encainide or flecainide in this study was 10 months. The applicability of the CAST results to other populations e, norpace cr 100mg. Considering the known proarrhythmic properties of Norpace or Norpace CR and the lack of evidence of improved norpace for any antiarrhythmic drug in patients without life-threatening arrhythmias, the use of Norpace or Norpace CR as well as other antiarrhythmic agents should be reserved for patients with life-threatening ventricular arrhythmias, norpace cr 100mg.
Hypotension has been 100mg primarily in patients with primary cardiomyopathy or inadequately compensated congestive heart failure. Norpace or Norpace CR should not be used in patients with uncompensated or norpace compensated congestive heart failure or hypotension unless the congestive heart failure or hypotension is secondary 100mg cardiac arrhythmia.
Patients with a history of heart failure may be treated 100mg Norpace or Norpace CR, but careful attention must be given to the maintenance of cardiac function, including optimal digitalization. If hypotension occurs or congestive heart failure worsens, Norpace or Norpace CR should be discontinued and, if necessary, restarted at a lower dosage only after adequate 100mg compensation has been established, norpace cr 100mg.
Q-T Prolongation As with other Type 1 antiarrhythmic drugs, prolongation of the Q-T interval corrected and worsening of the arrhythmia, including ventricular tachycardia and ventricular fibrillation, may occur, norpace cr 100mg. Patients who have evidenced prolongation of the Q-T interval in response to quinidine may be at particular risk. As with other Type 1A antiarrhythmics, disopyramide phosphate has been associated with torsade de pointes.
Hypoglycemia In rare instances significant lowering of blood-glucose values has been reported during Norpace administration, norpace cr 100mg.
The 100mg should be alert to this possibility, especially in patients with congestive heart 100mg, chronic malnutrition, norpace cr 100mg, hepatic, renal or other diseases, 100mg drugs e, norpace cr 100mg.
In these patients the blood-glucose levels should be carefully followed. Such use may produce serious negative inotropic effects, or may excessively norpace conduction. This should be considered particularly norpace patients with any degree of cardiac decompensation or those with a prior history thereof, norpace cr 100mg.
Patients receiving more than one antiarrhythmic drug must be carefully monitored. Heart Block If first-degree heart block develops in a patient receiving Norpace or Norpace CR, the dosage should be reduced. If the block persists despite reduction of dosage, continuation of the drug must depend upon weighing the benefit being obtained against the risk of higher degrees of heart block.
Development of second- or third-degree AV block or unifascicular, norpace cr 100mg, bifascicular, or trifascicular block requires discontinuation of Norpace or Norpace CR therapy, unless the ventricular rate is adequately controlled by a temporary or implanted ventricular pacemaker.
norpace
Norpace CR Still in Shortage, Anticipated Availability Announced
Anticholinergic Activity Because of its anticholinergic activity, disopyramide phosphate should not be used in patients with glaucoma, myasthenia gravis, or urinary retention unless adequate overriding measures are taken; these consist of the topical application of potent miotics e. Urinary retention may occur in patients of either sex as a consequence of Norpace or Norpace CR administration, but males with benign prostatic hypertrophy are at particular risk.
In patients with a family history of glaucoma, norpace cr 100mg, intraocular pressure should be measured before norpace Norpace or Norpace CR therapy. Disopyramide phosphate should be used with special care in patients with myasthenia gravis since its anticholinergic properties could precipitate a myasthenic crisis in such patients.
Conduction Abnormalities Care should be taken when prescribing Norpace or Norpace CR for patients with sick sinus syndrome bradycardia-tachycardia syndromeWolff-Parkinson-White syndrome WPWnorpace cr 100mg, or bundle branch block. The effect of disopyramide phosphate in barr pharmaceuticals methotrexate conditions is uncertain at present.
Cardiomyopathy Patients with myocarditis 100mg other cardiomyopathy may develop significant hypotension in response to the usual dosage of disopyramide phosphate, probably due to cardiodepressant mechanisms. Therefore, a loading dose of Norpace should not be given to such patients, and initial dosage and subsequent dosage adjustments should be made under close supervision see Dosage and Administration. Therefore Norpace dosage should clarithromycin 500mg la thuoc gi reduced in patients with impaired renal function see Dosage and Administration.
The electrocardiogram should be carefully monitored for prolongation of PR interval, evidence of QRS widening, or other signs of overdosage see Overdosage. Hepatic Impairment Hepatic impairment also causes an increase in the 100mg half-life of disopyramide.
Dosage should be reduced for patients with such impairment. The electrocardiogram should be carefully monitored for signs of overdosage see Overdosage. Patients with cardiac dysfunction have a higher potential for hepatic impairment; this should norpace considered when administering Norpace or Norpace CR.
Potassium Imbalance Antiarrhythmic drugs may be ineffective in patients with hypokalemia, and their toxic effects may be enhanced in patients with hyperkalemia. Therefore, potassium abnormalities should be corrected before starting Norpace or Norpace CR therapy.
Drug Interactions If phenytoin or other hepatic enzyme inducers are taken concurrently with Norpace or Norpace CR, lower plasma levels of disopyramide may occur. Monitoring of disopyramide plasma levels is recommended in such concurrent use to avoid ineffective therapy. Other antiarrhythmic drugs e. In healthy subjects, no significant drug-drug interaction was observed when Norpace was coadministered with either propranolol or diazepam.
Concomitant administration of Norpace and quinidine resulted in slight increases in plasma disopyramide levels and slight decreases in plasma quinidine levels. Norpace does not increase serum digoxin levels. Until data on possible interactions between verapamil and disopyramide phosphate are obtained, disopyramide should not be administered within 48 hours before or 24 hours after verapamil administration. Although potent inhibitors of cytochrome P 3A4 e.